Medical Affairs
Continued medical development of a drug or device post-launch is important to optimize medical use of the product and therefore also commercialization. The challenges are more diverse than the efficacy and safety demonstration focus of development. A product may have an effectiveness advantage that is hard to demonstrate in traditional development trials, all the challenges may be of healthcare delivery or cost effectiveness demonstration. Consequently, a prerequisite for developing medical affairs strategy and plans includes intricate knowledge of the respective characteristics of the many different ways of gathering and analyzing as well as disseminating data. 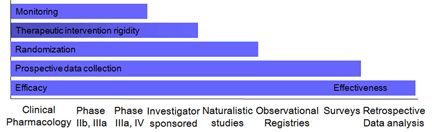 One example of this is the relationship between the different data gathering methodologies and whether the study primarily addresses efficacy or effectiveness as illustrated in the figure. There are many other differences as well including cost and operational burden. Similar to the different characteristics of data gathering, data dissemination can be done in many different ways including publications. Intricate knowledge of the various opportunities is important for optimized planning. IMD has experience in the whole range of both data gathering and dissemination.
One example of this is the relationship between the different data gathering methodologies and whether the study primarily addresses efficacy or effectiveness as illustrated in the figure. There are many other differences as well including cost and operational burden. Similar to the different characteristics of data gathering, data dissemination can be done in many different ways including publications. Intricate knowledge of the various opportunities is important for optimized planning. IMD has experience in the whole range of both data gathering and dissemination.